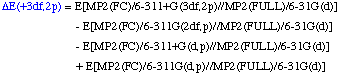G2 Theory
G2 theory aims to reproduce effectively QCISD(T)/6-311+G(3df,2p) energies
through a series of calculations at lower level. The G2 energy at 0 degrees Kelvin
E0(G2) is defined as:

The definition of the components being:
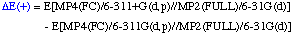
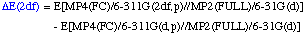
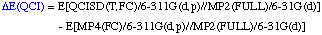
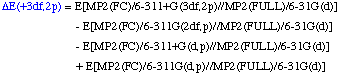

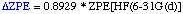
Comments:
- Open shell systems are treated using unrestricted wavefunctions (UHF, UMP2 . . )
- The higher level correction (HLC) is supposed to compensate remaining deficiencies
of the method. The A value has been chosen such that the energy of the hydrogen atom
is exact. The B value has been chosen such as to minimize the error for the predicted
heat of formation of 55 known molecules.
- The mean absolute deviation for the original G2 neutral set (125 reaction energies)
is 1.21 kcal/mol. The mean absolute deviation for the extended G2 neutral set (148 reaction
energies) is 1.58 kcal/mol.
- G2 energies at non-zero temperatures are obtained simply by adding thermochemical
corrections to the G2 energy at 0 K.
The necessary energies can be calculated most efficiently in the following sequence:
- Optimization and frequency calculation at the HF/6-31G(d) level of theory
- Optimization at the MP2(FULL)/6-31G(d) level of theory
- QCISD(T,FC)/6-311G(d,p)//MP2(FULL)/6-31G(d) single point
- MP4(FC)/6-311G(2df,p)//MP2(FULL)/6-31G(d) single point
- MP4(FC)/6-311+G(d,p)//MP2(FULL)/6-31G(d) single point
- MP2(FC)/6-311+G(3df,2p)//MP2(FULL)/6-31G(d) single point
All of these energies can be calculated step by step or in an automated manner using the G2 keyword
(here using water as an example):
#P G2
test154 G2 theory for water
0 1
O1
H2 1 r2
H3 1 r2 2 a3
r2=0.947323
a3=105.4974
The energy components will then be calculated in a sequence of different consecutive jobs.
The results will be listed at the very end of the output file in the following manner:
Temperature= 298.150000 Pressure= 1.000000
E(ZPE)= 0.020515 E(Thermal)= 0.023350
E(QCISD(T))= -76.276068 E(Empiric)= -0.024560
DE(Plus)= -0.010833 DE(2DF)= -0.037392
G1(0 K)= -76.328338 G1 Energy= -76.325502
G1 Enthalpy= -76.324558 G1 Free Energy= -76.345935
E(Delta-G2)= -0.008273 E(G2-Empiric)= 0.004560
G2(0 K)= -76.332051 G2 Energy= -76.329216
G2 Enthalpy= -76.328271 G2 Free Energy= -76.349648
DE(MP2)= -0.054454
G2MP2(0 K)= -76.330008 G2MP2 Energy= -76.327172
G2MP2 Enthalpy= -76.326228 G2MP2 Free Energy= -76.347605
The values given for the G2 Energy, the G2 Enthalpy and
the G2 Free Energy all refer to a temperature
of 298.15K. Values for different temperatures can be computed using the G2(Restart,ReadIso) keyword.
What is listed here as E(Empiric) corresponds to the HLC correction as defined for the G1 procedure.
The G2-type HLC energy is obtained by addition of what is listed as E(G2-Empiric) (corresponding to
1.14 mHartrees per valence electron pair).
Literature:
- L. A. Curtiss, K. Raghavachari, G. W. Trucks, J. A. Pople,
"Gaussian-2 theory for molecular energies of first- and second-row compounds"
J. Chem. Phys. 1991, 94, 7221 - 7230.
- L. A. Curtiss, J. E. Carpenter, K. Raghavachari, J. A. Pople
"Validity of additivity approximations used in Gaussian-2 theory"
J. Chem. Phys. 1992, 96, 9030 - 9034.
- L. A. Curtiss, K. Raghavachari, J. A. Pople,
"Gaussian-2 theory using reduced Møller-Plesset orders"
J. Chem. Phys. 1993, 98, 1293 - 1298.
- M. Glukhovtsev, A. Pross, M. McGrath, L. Radom,
"Extension of Gaussian-2 (G2) theory to bromine- and iodine-containing molecules:
Use of effective core potentials"
J. Chem. Phys. 1995, 103, 1878.
- L. A. Curtiss, P. C. Redfern, B. J. Smith, L. Radom,
"Gaussian-2 (G2) theory: Reduced basis set requirements"
J. Chem. Phys. 1996, 104, 5148.
- L. A. Curtiss, K. Raghavachari
"G2 Theory"
The Encyclopedia of Computational Chemistry, P. v. R. Schleyer (editor-in-chief),
John Wiley & Sons Ltd, Athens, USA, 1998, 2, 1104 - 1114.
last changes: 01.04.2008, AS
questions & comments to: axel.schulz@uni-rostock.de